Abstract
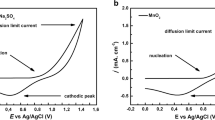
Nanostructured molybdenum oxide having a particle size in the range of 30–80 nm was prepared by potentiodynamic electrodeposition method, and the effects of H2SO4 concentration on its capacitive behavior were studied by cyclic voltammetry, galvanostatic discharge, and electrochemical impedance spectroscopy. Poor to fair capacitive behaviors were witnessed depending on the electrolyte concentration and conditions of charge/discharge. Increasing acid concentration to 0.02 M had favorable effect, while beyond that, the effect was detrimental. Capacitance around 600 F g−1 was recorded in the potential range of 0 to −0.55 V vs. Ag/AgCl.





Similar content being viewed by others
References
Bailar JC, Emeléus HJ, Nyholm SR, Trotman-Dickenson AF (eds) (1973) Comprehensive inorganic chemistry vol 3. Pergamon, Oxford
Heracleous E, Lee AF, Vasalos IA, Lemonidou AA (2003) Catal Lett 88:47. doi:10.1023/A:1023534816277
Ferroni M, Guidi V, Martinelli G, Sacerdoti M, Nelli P, Sberveglieri G (1998) Sens Actuator B 48:285. doi:10.1016/S0925-4005(98) 00057-4
Imawan C, Steffes H, Solzbacher F, Obermeier E (2001) Sens Actuator B 77:346. doi:10.1016/S0925-4005(01) 00732-8
Granqvist CG (1995) Handbook of inorganic electrochromic materials. Elsevier, Amsterdam
Shembel E, Apostolova R, Nagirny V, Kirsanova I, Grebenkin P, Lytvyn P (2005) J Solid State Electrochem 9:96. doi:10.1007/s10008-004-0565-2
Yebka B, Julien C, Nazri GA (1999) Ionics 5:236. doi:10.1007/BF02375846
Christian PA, Carides JN, DiSalvo FJ, Waszczak V (1980) J Electrochem Soc 127:2315. doi:10.1149/1.2129404
McEvoy TM, Stevenson KJ (2003) Anal Chim Acta 496:39. doi:10.1016/j.aca.2002.10.001
McEvoy TM, Stevenson KJ (2004) J Mater Res 19:429. doi:10.1557/jmr.2004.19.2.429
McEvoy TM, Stevenson KJ, Hupp JT, Dang X (2003) Langmuir 19:4316. doi:10.1021/la027020u
Pathan HM, Min SK, Jung KD, Joo OS (2006) Electrochem Commun 8:273. doi:10.1016/j.elecom.2005.11.022
Guerfi A, Dao LH (1989) J Electrochem Soc 136:2435. doi:10.1149/1.2097408
Nagirnyi VM, Apostolova RD, Baskevich AS, Shembel EM (2004) Russ J Appl Chem 77:71. doi:10.1023/B:RJAC.0000024579.88110.c3
Więcek B, Twardoch U (2004) J Phys Chem Solids 65:263. doi:10.1016/j.jpcs.2003.08.022
Prasad KR, Koga K, Miura N (2004) Chem Mater 16:1845. doi:10.1021/cm0497576
Prasad KR, Miura N (2004) J Power Sources 135:354. doi:10.1016/j.jpowsour.2004.04.005
Prasad KR, Miura N (2004) Electrochem Commun 6:1004. doi:10.1016/j.elecom.2004.07.017
Prasad KR, Miura N (2004) Electrochem Commun 6:849. doi:10.1016/j.elecom.2004.06.009
Więcek B, Kępas-Suwara A (2007) Pol J Chem 81:129
Takasu Y, Ohnuma T, Sugimoto W, Murakami Y (1999) Electrochemistry 67:1187
Farsi H, Gobal F, Raissi H, Moghiminia S (2009) On the psuedocapacitive behavior of nanostructured molybdenum oxide. J Solid State Electrochem. doi:10.1007/s10008-009-830-5
García-Cañadas J, Mora-Seró I, Fabregat-Santiago F, Bisquert J, Garcia-Belmonte G (2004) J Electroanal Chem 565:329. doi:10.1016/j.jelechem.2003.10.027
Conway BE (1999) Electrochemical supercapacitors: scientific fundamentals and technological applications. Kluwer Academic/Plenum, New York
Lin C, Ritter JA, Popov BN, White RE (1999) J Electrochem Soc 146:3168. doi:10.1149/1.1392450
Farsi H, Gobal F (2007) J Solid State Electrochem 11:1085. doi:10.1007/s10008-006-0242-8
Farsi H, Gobal F (2009) J Solid State Electrochem 13:433. doi:10.1007/s10008-008-0576-5
Pico F, Ibañez J, Centeno TA, Pecharroman C, Rojas RM, Amarilla JM, Rojo JM (2006) Electrochim Acta 51:4693. doi:10.1016/j.electacta.2005.12.040
Sugimoto W, Iwata H, Yokoshima K, Murakami Y, Takasu Y (2005) J Phys Chem B 109:7330. doi:10.1021/jp044252o
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farsi, H., Gobal, F., Raissi, H. et al. The pH effects on the capacitive behavior of nanostructured molybdenum oxide. J Solid State Electrochem 14, 681–686 (2010). https://doi.org/10.1007/s10008-009-0828-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-009-0828-z